Reaction kinetic studies in a plug flow reactor
The following data are obtained in an ideal tubular flow reactor for the gaseous pyrolysis of acetone at 520°C and 1 atm. The reaction is
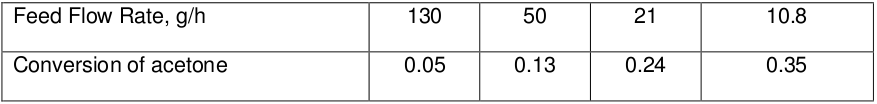
The feed consists of pure acetone, and the reactor is a 3.3 cm i.d. pipe of 80 cm length. Consider that the reaction mixture obeys ideal gas law and reaction follows second-order kinetics. Find the reaction velocity constant.
